Harnessing the Power of Natural Killer Cells for Cancer Immunotherapy

The immune system is our body’s biological defense system against a variety of pathogens and is separated into the innate and adaptive branches. Our innate immune system is the body’s first line of defense, activating immediately or within hours of a foreign antigen’s appearance in the body with natural killer (NK) cells playing a key role in immunosurveillance. Unlike T (and B) cells, NK cells are able to recognize “non-self” cells without the need for prior sensitization and major histocompatibility complex (MHC) mechanisms, which allows them to execute rapid immune responses. This broad cytotoxicity against cancer and virus-infected cells without MHC restriction is of great interest for cancer immunotherapies.
Because NK cells are essential to mounting immune responses against cancer cells, there are a number of strategies to enhance their antitumor activity in cancer immunotherapy. Techniques to increase endogenous NK cell activation through cytokine or checkpoint inhibition, increasing the number of active NK cells through ex vivo expansion or genetic modification of NK cells aim to increase their clinical efficacy in the fight against cancer. Pre-clinical and clinical studies have demonstrated the safety and efficacy of treatment using both unmodified and the engineered forms of allogeneic NK cells against various hematological malignancies and solid tumors and many NK cell-based clinical trials are currently ongoing1.
Natural Killer Cells
First identified in 1975, NK cells are large granular lymphocytes and potent cytolytic effectors of the innate immune system. NK cells are descended from the common lymphoid progenitor, which also gives rise to B and T lymphocytes and dendritic cells. Human NK cells are characterized as CD3−CD56+CD16+. They can be further subdivided based on their CD56 expression levels, CD56bright and CD56dim with each population contributing distinct yet integral functions during the human NK immune response.
NK cells induce apoptosis in cancer or infected cells by releasing cytolytic proteins like granzymes and perforin as well as a host of cytokines that recruit other immune cells to the site of infection2. Their ability to target cells independent of the MHC complex makes them uniquely suited to eliminating harmful cells that lack or downregulate their MHC1 markers to evade detection by other immune cells (like T cells)2,3.
An important distinction of NK cells is that their activation is not controlled by antigen specificity like T cells. Rather, their broader activation is regulated by the balance between activating and inhibitory surface receptors. For example, when cellular stress or DNA damage occurs during viral infection or tumor formation, an upregulation of “stress ligands” on transformed cells binds to activating NK receptors which initiates target cell lysis. Conversely, when inhibitory signals are predominant, the NK cell is not activated, as is the case when they encounter “self” cells.
However, this mode of target cell recognition lacks specificity, and is a key challenge in harnessing NK cells for cancer immunotherapy. Several pharmacological and genetic strategies to enhance their specificity for tumors are under investigation:
- The use of immune stimulants like cytokines to improve NK cell efficacy.
- Targeting NK cell immune checkpoints through checkpoint inhibition.
- The use of bi- or tri-specific killer cell engagers to activate NK cells.
- Adoptive transfer of activated NK cells expanded ex vivo.
- Genetic modification of NK cells to express with chimeric antigen receptors (CARs) to enhance their antitumor activity.
Strategies to Enhance Endogenous NK Cell Activity
Cytokines (Figure 1A). There are a number of biological molecules dubbed NK cell “enhancers” which increase their endogenous activity to improve anti-tumor responses through upregulation of cytolytic, secretory, and proliferative functions. IFNα, Interleukin (IL)-2, IL-12, IL-15 and IL-18 play important roles for the survival, proliferation and activation of NK cells4.
IL-2 is a cytokine commonly used to boost cytotoxicity of NK cells, but, high-dose IL-2 cancer immunotherapy can cause severe side effects, including vascular leakage and organ injury5. IL-2 also activates CD4+ regulatory T cells (Tregs), which preferentially bind to and deplete IL-2 through their high-affinity IL-2Rαβγ receptors thus suppressing NK cell activation to eliminate infected cells4. As such, other NK cell-stimulatory cytokines like IL-15 are better candidates for cancer immunotherapy.
IL-15 is a homeostatic, proinflammatory cytokine for NK and T cells, required for NK-cell development. Unlike IL-2, IL-15 potentiates NK cell antitumor activity without promoting Treg function and has shown promising results as a cancer therapeutic in human clinical trials. Subcutaneous rhIL-15 treatment was well tolerated, producing substantial increases in circulating NK and CD8+ T cells in refractory solid tumor cancer patients6.
Monoclonal antibodies & Checkpoint Inhibition (Figure 1B). Tumor‐targeting monoclonal Abs (mAbs) to activate endogenous NK cells, are widely used as anticancer therapeutics with many of these antibodies approved by the FDA7. mAbs bound to specific tumor antigens via the Fab fragment can trigger NK-mediated effector functions like ADCC through binding of IgG FcγRIIIA on the NK activating receptor CD16A. mAbs including anti-CD20 (rituximab), anti–human epidermal growth factor receptor 2 (trastuzumab), anti-CD52 (alemtuzumab), anti–epidermal growth factor receptor (cetixumab), and anti-CD38 (daratumumab), have been developed to target specific tumor antigens8.
The principle behind immune checkpoint blockade is to use mAbs targeted against inhibitory NK cell receptors to enhance anti-tumor activity. The mAb-mediated blockade of NK cell-inhibitory receptors can revert the functional inhibition of these cells and restore an effective antitumor cytotoxic activity, possibly leading to durable tumor regression9. KIR and NKG2A are well-studied immune-checkpoints of NK cells and mAbs agonists against these receptors have shown preclinical promising results.
BiKEs /TriKES (Figure 1C). Bi-specific or tri-specific killer cell engagers (BiKEs or TriKEs) are artificial small molecules (50-75kDa) comprised of a variable heavy and variable light chain (VH and VL) against CD16 (an NK cell-activating receptor) linked to the single-chain variable fragments (scFv) of either one (BiKEs) or two (TriKEs) variable regions from tumor-associated antigen (TAA) specific Abs9,10. BiKEs and TriKEs form a link between the tumor and NK cells through the simultaneous binding of their surface receptors, which enhances NK cell-mediated killing through ADCC.
BiKEs and TriKEs have been generated to engage CD16 on the NK cell and a number of TAAs including CD20 and CD19 on B cell Non-Hodgkin’s lymphomas, CD33 or CD33 and CD123 on acute myelogenous leukemia (AML) and more10. Another version of TriKEs that have found utility are BiKEs directed against a specific tumor antigen with the addition of IL-15 to form a cytokine-antibody hybrid molecule. IL-15 acts to enhance NK cell priming, proliferation while the antibody fragment provides the specificity. For example, the CD16-CD33-IL-15 TriKE targeted against AML showed better NK cell proliferation survival compared to the CD16-CD33 BiKE10.
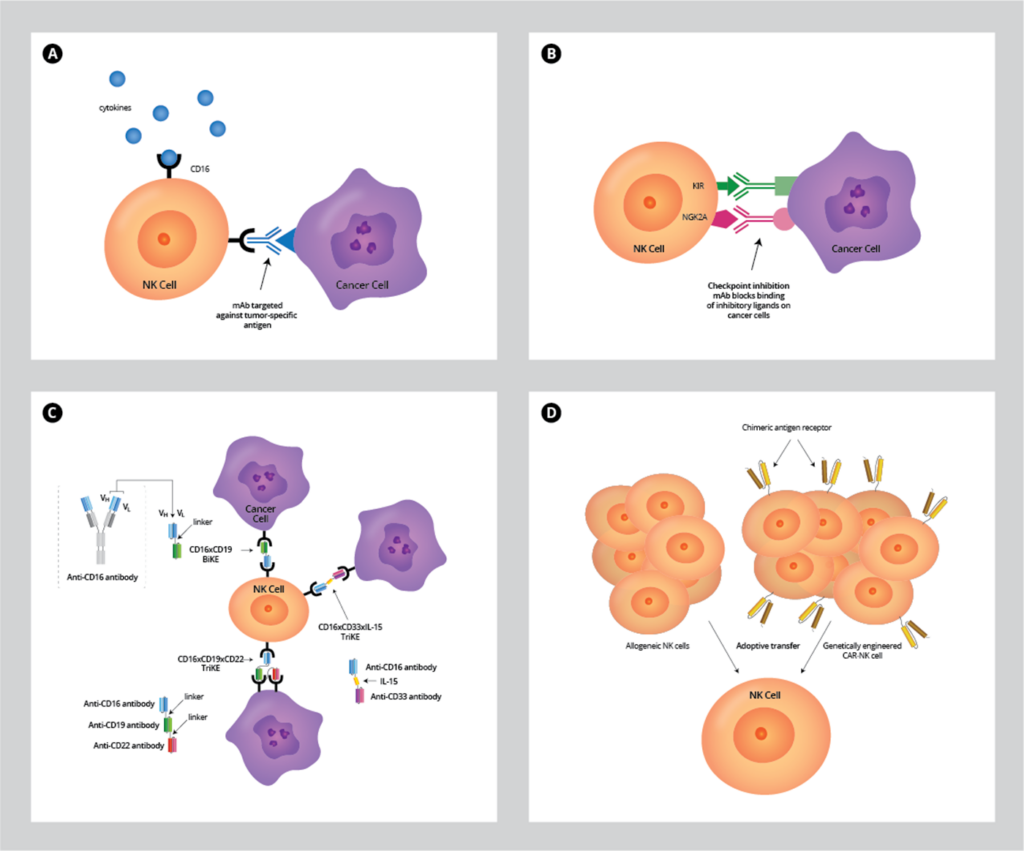
Figure 1. Approaches to enhance antitumor activity of NK cell therapy.
A. Treatment with cytokines like IL-15 or mAbs directed against specific tumor antigens, which increases NK‐cell activation in vivo.
B. Checkpoint inhibition: mAb block KIR and NKG2A receptors from being activated by inhibitory ligands to release inhibitory signals and activate NK cells.
C. Bispecific (BiKEs) or trispecifc (TriKES) engagers that link CD16 on NK cells to one or two tumor-specific antigens to promote their activation, respectively. Specialized TriKE molecules containing IL-15 cytokine with antigen-specificity promotes NK priming and proliferation as well as activation.
D. Adoptive transfer of allogeneic NK cells obtained from donor material as well as genetically modified CAR-NK cells have shown promising clinical results to boost NK activity for several oncological indications.
Adoptive Transfer of Allogeneic NK Cells
Both autologous and allogeneic NK cells have been proven to be safe and tolerable in adoptive cell therapy. Results indicate that allogeneic NK cells are better candidates for cancer immunotherapy because they exhibit better antitumor activity than autologous NK cells11. Additionally, they are attractive for adoptive cell transfer in cancer immunotherapy since they do not cause graft vs. host disease (GvHD). Indeed, clinical studies have demonstrated safety and efficacy of allogeneic infusions of NK cells for immunotherapy in patients with AML, myelodysplastic syndrome (MDS) and solid tumors12,13. Most NK-cell-based treatments are based on infusing alloreactive donor peripheral blood mononuclear cells (PBMC)-derived NK cells activated ex vivo. Other NK cell sources include umbilical cord blood (UBC), clonal NK cell lines such as NK-92 and NK cells differentiated from human embryonic stem cells (hESCs), as well as induced pluripotent stem cells (hiPSCs) (Figure 1D).
Primary NK Cells
Primary NK cells can be obtained from both peripheral blood mononuclear cells (PBMCs) and umbilical cord blood (UCB) cells. They can be difficult to isolate, purify, and transduce, often producing a heterogeneous cell population that expands poorly14. Commonly, magnetic antibody separation is used to isolate the NK population from other immune cells prior to expansion and activation.
Cell Lines
NK cell lines such as NK-92, NKL and NKG are candidates for immunotherapies. These cell lines are derived from patients with leukemia or lymphoma, dependent on IL-2 for the proliferation and antitumor activity. The lack of KIR expression on the cell surface and inability to kill through ADCC due to low or absent CD16 expression greatly differentiates this cell line from primary NK cells15.
One advantage of these clonal cell lines is they are easily culture-expanded to produce large numbers of NK cells for infusion. The most extensively studied is the NK-92 cell line, which has shown higher cytotoxicity against a number of primary and established tumor cells compared to activated unmodified NK cells4. However, safety concerns due to its origin from an NK lymphoma necessitates irradiation of these cells prior to infusion into patients. While studies do show that their cytotoxicity is not diminished as a result of irradiation, their inability to expand in vivo and short lifespan (<7 days) in rapid clearance of the cells, which can impact their therapeutic effect, likely requiring multiple rounds of infusion per patient15.
Pluripotent stem cell-derived NK Cells
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) can offer another renewable and potentially better source of NK cells. These undifferentiated cells can be culture expanded and subsequently differentiated into NK cells. However, some studies indicate that NK cells derived from these cell sources may have lower cytotoxicity compared to the more mature, PBMC-derived NK cells, for example15. Another challenge is identifying the optimal hESC/iPSC cell line and differentiation protocol to obtain larger numbers of therapeutic NK cells.
Chimeric Antigen Receptor (CAR)-NK Cells
The clinical success of CAR-T cells has led researchers to genetically modify NK cells with CARs to enhance their tumorigenicity. NK cells can be transduced to express chimeric antigen receptors (CARs) for cancer retargeting in much the same way as in T cells (CAR-T cell therapy ) (Figure 1D). The CAR constructs are composed of an extracellular antigen-recognition ScFv connected to a transmembrane domain followed by an intracellular signaling/activation CD3ζ domain with one or more costimulatory domains that provides a signal to activate NK cells. In addition to engineered cancer-homing receptors, CAR-expressing NK cells retain their native NK cell receptor-dependent mechanisms, making them potent effectors for immunotherapy16.
Advantages over CAR-T Cells
CAR-NK cells have several advantages over CAR-T cells. First, CAR-NK cells retain their native receptor functions, allowing them to capture tumor cells that try to evade T cell detection by downregulating the CAR-specific antigen (i.e. CD19). Also, the short-term persistence of infused CAR-NK cells mitigates undesirable side effects observed with CAR-T cells like GvHD and cytokine release syndrome. This “off the shelf” product approach could greatly decrease time, cost and increase accessibility to CAR-NK therapy.
Challenges
While the preclinical and clinical data thus far has been promising, CAR-NK cell-based immunotherapy is still in its infancy in comparison to CAR-T and there are a number of unknowns and challenges to address. NK cells are more difficult to genetically manipulate and expand in culture to reach the numbers required for infusion, and their limited in vivo persistence all impact their success for cancer immunotherapy.
Another hurdle is the short lifespan of infused CAR-NK cells (1-2 weeks post-infusion) and limited expansion in vivo, which means that while they are less likely to cause off-target effects (as observed with CAR-T cells), it may prevent any long-term durability of their therapeutic effect, potentially leading to cancer relapse15,16. Therefore, identifying the best source of NK cells to arm with CARs, improving their ex vivo expansion, increasing their in vivo durability, determining the optimal vector system, and designing CARs that work optimally for NK cells to provide the most potent therapeutic effect while maintaining safety will be paramount to success and are all areas of active research1.
There is still much to learn about CAR-NK cell mechanisms, but they could be a valued addition to the immunotherapy toolkit, potentially as a complementary therapy to CAR-T cells. Because NK cells have an intrinsic cytotoxicity towards tumor cells that is independent of the CARs, CA- NK cells may be better at capturing tumor cells that have evolved mechanisms to evade the T cells4. Therefore, a combinatory approach with both CAR-T and CAR-NK cells may lead to better cancer clearance than with CAR-T cells alone.
Concluding Remarks
There are currently a number of approaches under investigation to exploit the NK cells’ antitumor immune functions in cancer immunotherapy. Better understanding of how NK cells respond to different tumor types through their ligands and receptors will aid in identifying approaches that can be used therapeutically12. Their impressive safety profile over CAR-T cells could potentially broaden their clinical use but more work needs to be done to identify better tumor targets, and more. With all these facets of research, NK cells are poised to make a considerable impact on the future of cancer treatment.
AllCells Product Offerings for NK Cell Research
AllCells offers high quality primary cells from a diverse, recallable donor pool. AllCells also offers negative or positively selected CD56+ Natural Killer (NK) cells from peripheral blood using immunomagnetic microbeads for ease of use.
Footnotes
- Natural killer cells for cancer immunotherapy: a new CAR is catching up. EBioMedicine. 2019;39:1‐2. doi:10.1016/j.ebiom.2019.01.018
- Topham NJ, Hewitt EW. Natural killer cell cytotoxicity: how do they pull the trigger?. Immunology. 2009;128(1):7–15. doi:10.1111/j.1365-2567.2009.03123.x
- Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther. 2017;25(8):1769–1781. doi:10.1016/j.ymthe.2017.06.012
- Littwitz-Salomon E, Malyshkina A, Schimmer S, Dittmer U. The Cytotoxic Activity of Natural Killer Cells Is Suppressed by IL-10+ Regulatory T Cells During Acute Retroviral Infection. Front Immunol. 2018;9:1947. Published 2018 Aug 27. doi:10.3389/fimmu.2018.01947
- Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol. 2019;10:1205. Published 2019 May 31. doi:10.3389/fimmu.2019.01205
- Miller JS, Morishima C, McNeel DG, et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (rhIL15) in Adults with Advanced Solid Tumors. Clin Cancer Res. 2018;24(7):1525‐1535. doi:10.1158/1078-0432.CCR-17-2451
- Li Y, Sun R. Tumor immunotherapy: New aspects of natural killer cells. Chin J Cancer Res. 2018;30(2):173‐196. doi:10.21147/j.issn.1000-9604.2018.02.02
- Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front Immunol. 2015;6:605. Published 2015 Nov 30. doi:10.3389/fimmu.2015.00605
- Barrow AD, Colonna M. Exploiting NK Cell Surveillance Pathways for Cancer Therapy. Cancers (Basel). 2019;11(1):55. Published 2019 Jan 8. doi:10.3390/cancers11010055
- Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol Biol. 2016;1441:333‐346. doi:10.1007/978-1-4939-3684-7_28
- Yang C, Yue Li Y, Yang Y, Chen Z. Overview of Strategies to Improve Therapy against Tumors Using Natural Killer Cell. J of Immun. Res. 2020; Vol 2020, Article ID 8459496. doi: 10.1155/2020/8459496
- Lupo KB, Matosevic S. Natural Killer Cells as Allogeneic Effectors in Adoptive Cancer Immunotherapy. Cancers (Basel). 2019;11(6):769. doi:10.3390/cancers11060769
- Chen Z, Yang Y, Liu LL, Lundqvist A. Strategies to Augment Natural Killer (NK) Cell Activity against Solid Tumors. Cancers (Basel). 2019;11(7):1040. doi:10.3390/cancers11071040
- Mehta RS, Rezvani K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front Immunol. 2018;9:283. doi:10.3389/fimmu.2018.00283
- Pfefferle A, Huntington ND. You Have Got a Fast CAR: Chimeric Antigen Receptor NK Cells in Cancer Therapy. Cancers (Basel). 2020;12(3):706. Published 2020 Mar 17. doi:10.3390/cancers12030706
- Hu Y, Tian ZG, Zhang C. Chimeric antigen receptor (CAR)-transduced natural killer cells in tumor immunotherapy. Acta Pharmacol Sin. 2018;39(2):167–176. doi:10.1038/aps.2017.125