GMP Leukopaks for Allogeneic Cell Therapies

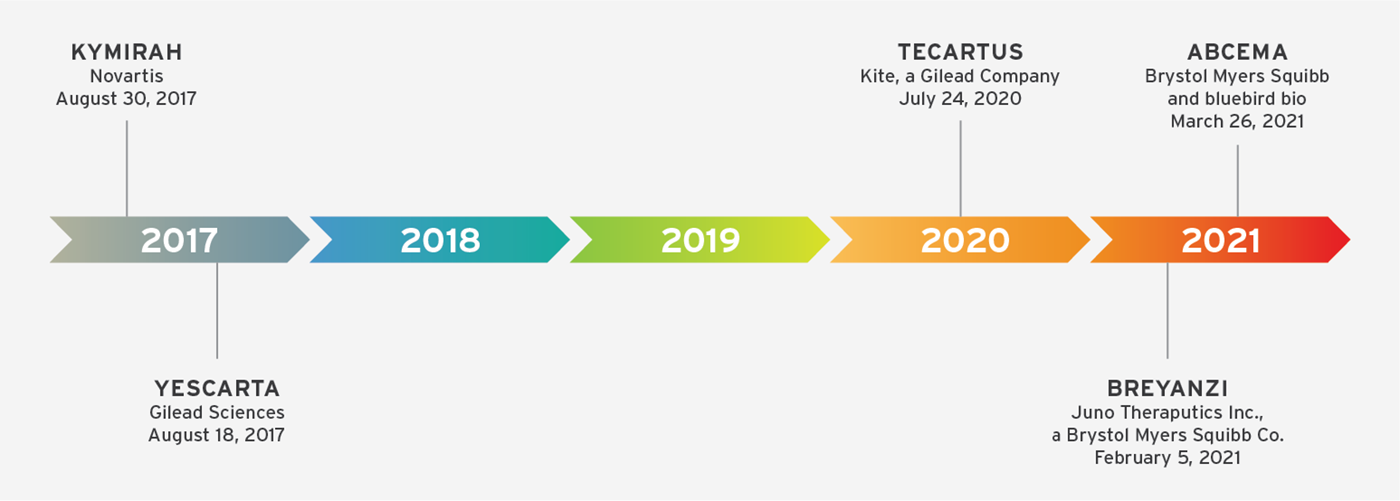
Adoptive cell therapies (ACTs), which is a treatment based on transferring cells (most often immune cells) into a patient, have revolutionized the standard of care to treat a broad range of diseases. It has perhaps made the most impact in cancer immunotherapy where the successes of chimeric antigen receptor (CAR) T cell therapies to treat relapsed and refractory CD19+ B cell malignancies has resulted in exponential growth in the field, now with 5 FDA-approved treatments since the landmark approvals in 2017.
ADOPTIVE CELL THERAPY IS MORE THAN CAR-T
While CAR-T immunotherapies have garnered the lion’s share of interest (for good reason), ACT actually encompasses a number of other immune cell types and strategies, some of which will be summarized here.
Engineering the native T cell receptor (TCR) on the surface of T lymphocytes is another ACT approach to targeting tumor-specific antigens. With TCR-T cell therapy, MHC molecules can present antigen fragments from cell surface or intracellular proteins in comparison to CAR-Ts, which can only recognize cell surface targets. While this enables TCR-T cells to act against a wider scope of targets, MHC restriction remains a limitation. In one clinical trial, modified TCR-T cells targeted against a common cancer antigen, New York esophageal squamous cell carcinoma (NY-ESO-1), were used to treat multiple myeloma patients. 80% of patients with multiple myeloma had a good clinical response, and 70% of them had a complete or near-complete response1.
Another subset of T cells that has garnered interest for ACT are Immunosuppressive regulatory T cells (Tregs) for the treatment of autoimmune diseases like type 1 diabetes. Defects in Tregs function leads to unchecked proliferation/activation of autoreactive T cells that kill off insulin-producing pancreatic β cells, resulting in diabetes onset. Recent phase l clinical trials have evaluated the safety and efficacy of adoptive transfer of polyclonal Tregs expanded ex vivo (NCT01210664, ISRCTN06128462) to restore Treg function in diabetic patients2.
The success of chimeric receptors for cancer therapy has also been expanded into other lymphoid immune cell types, such as γδ T cells3,4, natural killer T (NKT) cells3, natural killer (NK) cells5, macrophages4 and dendritic cells6. Many of these cell types exert a broader tumor-killing response over standard CAR-T cells, which may be advantageous for solid tumor cancers where CAR-T efficacy has been limited. Additionally, these lymphocyte subsets may provide an avenue toward the development of “off-the-shelf” allogeneic cell products4. High-quality GMP leukapheresis material is needed to support these development efforts.
ALLOGENEIC CGT THERAPIES
Both autologous (patient-derived cells) and allogeneic (donor cells) therapies exist in the ACT space, each with their own advantages and disadvantages. While autologous therapies are more advanced, analysis of trends in immunotherapy development indicate there is a larger percentage of allogeneic therapies in pre-clinical and Phase I primed for rapid progression through the clinical pipeline and eventual regulatory approval and commercialization.
Allogeneic ACT, where cells are obtained from healthy donors, are more cost-effective, easily scalable for commercial production and may provide a higher quality product. Here, the quality of the donor leukapheresis material is the most critical determinant of CGT success. However, a key challenge is that there is inherent donor variability in the population, where circadian fluctuations7, gender8,9,10, age7, ethnicity7, BMI11,12 and lifestyle differences (i.e., smoking12) can affect the quality of the leukapheresis collection. This is at odds with regulatory authorities who want to treat cell-based therapeutics like traditional pharmaceuticals where the end products show little variability and are highly consistent. Under these unique circumstances, where the cells are the therapeutic, process control becomes all the more important. It is essential to reduce process-related variability, which includes controlling leukapheresis collection to mitigate biological sources of variability and heterogeneity.
ALLCELLS’ GMP LEUKOPAKS
With a lack of standardization in the field for starting raw cellular materials, choosing the right supplier with expertise in GMP regulatory requirements for donor selection and tissue processing removes potential roadblocks and delays. Establishing trust in your partner to meet your evolving program needs becomes critical. AllCells offers GMP Leukopaks collected in our FDA-approved facilities and each batch undergoes stringent quality review with extensive collecting and QC testing documentation to meet GMP guidelines. Our recallable and reliable clinical-grade donors are recruited, screened and selected from the industry’s largest and most diverse repository in compliance with FDA current Good Tissue Practices (cGTP) regulations under 21 CFR 1271. We have over 5 years in the provision of GMP materials and are dedicated to meet the growing volume requirements and tight timelines faced by our CGT development partners without compromising on quality to ensure long-term success.
Learn more about AllCells’ GMP Leukopaks or contact us at orders@allcells.com to speak to an AllCells team member.
REFERENCES
- Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21(8):914-921. doi:10.1038/nm.3910
- Volfson-Sedletsky V, Jones A 4th, Hernandez-Escalante J, Dooms H. Emerging Therapeutic Strategies to Restore Regulatory T Cell Control of Islet Autoimmunity in Type 1 Diabetes. Front Immunol. 2021;12:635767. Published 2021 Mar 18. doi:10.3389/fimmu.2021.635767
- Patel S, Burga RA, Powell AB, et al. Beyond CAR T Cells: Other Cell-Based Immunotherapeutic Strategies Against Cancer. Front Oncol. 2019;9:196. Published 2019 Apr 10. doi:10.3389/fonc.2019.00196
- Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81(5):1201-1208. doi:10.1158/0008-5472.CAN-20-2990
- Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021;14(1):7. Published 2021 Jan 6. doi:10.1186/s13045-020-01014-w
- Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10(1):5408. Published 2019 Nov 27. doi:10.1038/s41467-019-13368-y
- Louati N, Rekik T, Menif H, Gargouri J. Blood lymphocyte T subsets reference values in blood donors by flow cytometry. Tunis Med. 2019;97(2):327-334.
- Sarkodee-Adoo C, Taran I, Guo C, et al. Influence of preapheresis clinical factors on the efficiency of CD34+ cell collection by large-volume apheresis. Bone Marrow Transplant. 2003;31(10):851-855. doi:10.1038/sj.bmt.1704034
- Lee BW, Yap HK, Chew FT, Quah TC, Prabhakaran K, Chan GS and al. Age- and sex-related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry 1996; 26: 8-15.
- Chang, WJ, Tan GB, and Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin. Diagn. Lab Immunol 2004; 11: 168-73.
- Chen J, Burns KM, Babic A, et al. Donor body mass index is an important factor that affects peripheral blood progenitor cell yield in healthy donors after mobilization with granulocyte-colony-stimulating factor. Transfusion. 2014;54(1):203-210. doi:10.1111/trf.12238
- Furuncuoğlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300-1306.