Improved Process for Consistent, High-quality Starting Material Minimizing Downstream Variability
Bone marrow aspirate is a rich source of CD34+ hematopoietic stem and progenitor cells (HSPCs), mesenchymal stem/stromal cells (MSC) and mononuclear cells (MNCs) (Fig1). HSPC-based cell therapies are currently being developed with applications in oncology, hemoglobinopathies, neurological diseases and others while MSC-based therapies are being developed in the areas of inflammation and tissue injury. With the leaps and strides in the advancements in the cell and gene therapy world, a reliable supplier of bone marrow aspirate capable of supporting the entire journey from research to commercialization with high quality products is crucial to successful clinical translation.
AllCells is the leading provider of human bone marrow aspirate that is withdrawn from bilateral punctures of the posterior iliac crests of our healthy donors. This invasive procedure requires extensive clinical expertise, IRB oversight to ensure donor comfort and safety as well as a stringently controlled collection process that ultimately determines the product quality.
Typical Bone Marrow Aspirate Composition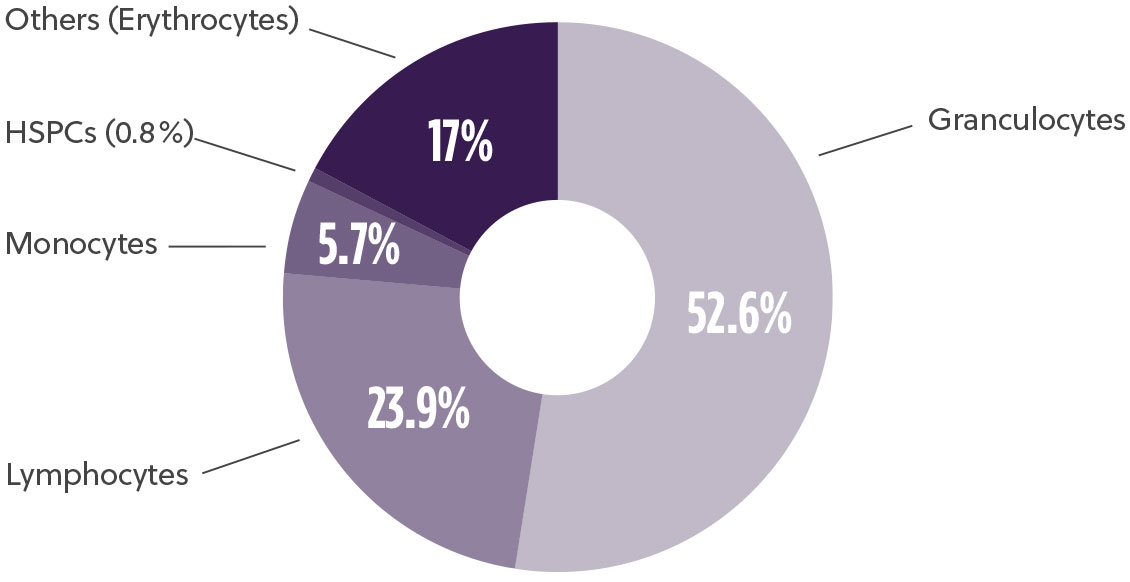
Applications:
- Hematopoiesis & Hematopoietic microenvironment studies
- In vitro functional assays (colony forming assays, long-term culture initiating-cell assays (LTC-IC), etc)
- Progenitor cell proliferation & differentiation, Stem cell expansion
- Cell and gene therapy research and productization
- Toxicology
- Drug discovery/screening, lead optimization
There are many factors that influence cell yield and subpopulation breakdown In bone marrow aspiration samples, from individual donor attributes to collection techniques. An optimised and standardized harvesting technique that minimizes contamination by unwanted cell types (i.e., granulocytes) during the aspiration reduces variability and ensures a higher quality product that meets minimal cell count and viability criteria.
We have recently implemented a proprietary process change to improve our bone marrow aspiration protocol that delivers a higher viable Total Nucleated Cells (TNCs) and CD34+ yield from a single donor ensuring sufficient cells for your downstream workflow, minimizing experimental variability, compared to our previous protocol. Additionally, this method reduces cell contamination from red blood cells and granulocytes. Overall, these are attributed to a higher quality bone marrow aspirate product.
More Donor Samples Meeting Criteria
We analyzed a total of 57 bone marrow aspirate samples collected using either the old or new method (Table 1). Our data indicate that the new method resulted in improved total nucleated cell (TNC) yield, with more collections meeting a higher cell count. Our new improved method results in a 72% and 150% increase in donor samples with more than 15M TNCs/mL and 20M TNCs/mL, respectively, as compared to the old method.
Table 1
| Number of Samples with: | Previous Method (n=28) | New Method (n=29) | Conclusion |
|---|---|---|---|
| ≥15 million TNCs/ml | 50% | 86% | 72% increase in donor samples with more than 15 million TNCs/mL |
| ≥20 million TNCs/ml | 18% | 45% | 150% increase in donor samples with more than 20 million TNCs/mL |
A More Targeted Aspiration with Minimized Peripheral Blood Cell Contamination
The new method demonstrated a 20% increase in TNC cell count (M/mL) (Fig2a) as well as a 17% increase in the % CD34+ cell content (Fig2b). At the same time, analysis of the subpopulation breakdown within the samples showed increased mononuclear cell (MNC) yield with concomitant decrease in contaminating neutrophils (Fig2c). This indicates that the overall increase in TNC was attributed to a higher proportion of MNCs and not a result of contaminating cell populations. Our method also resulted in a higher consistency in the TNCs counts between samples (results not shown here).
Fig 2a. Average Total Nucleated Cells (TNCs)
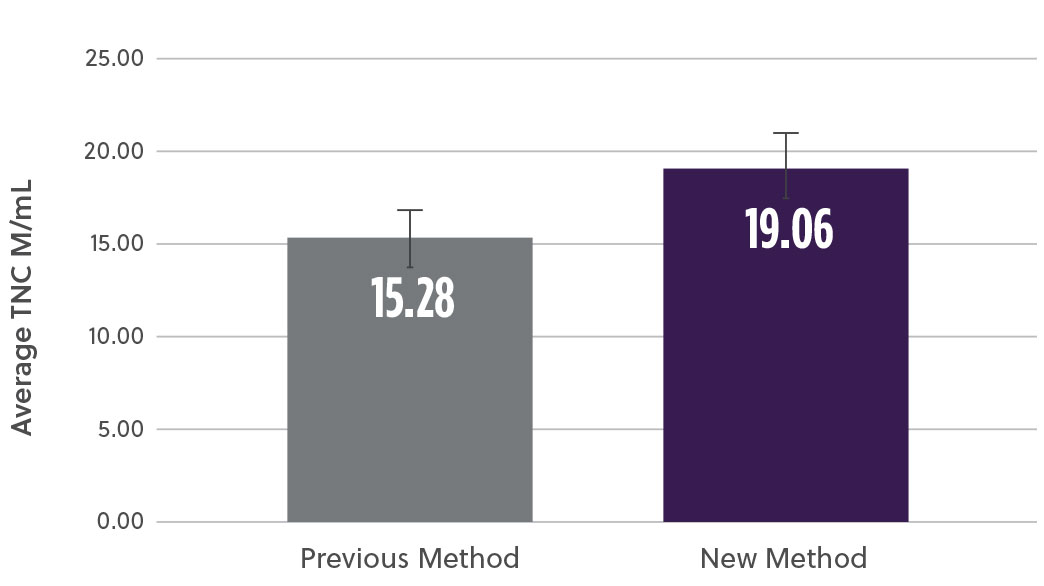
Fig 2b. % CD34+ Cells by ISHAGE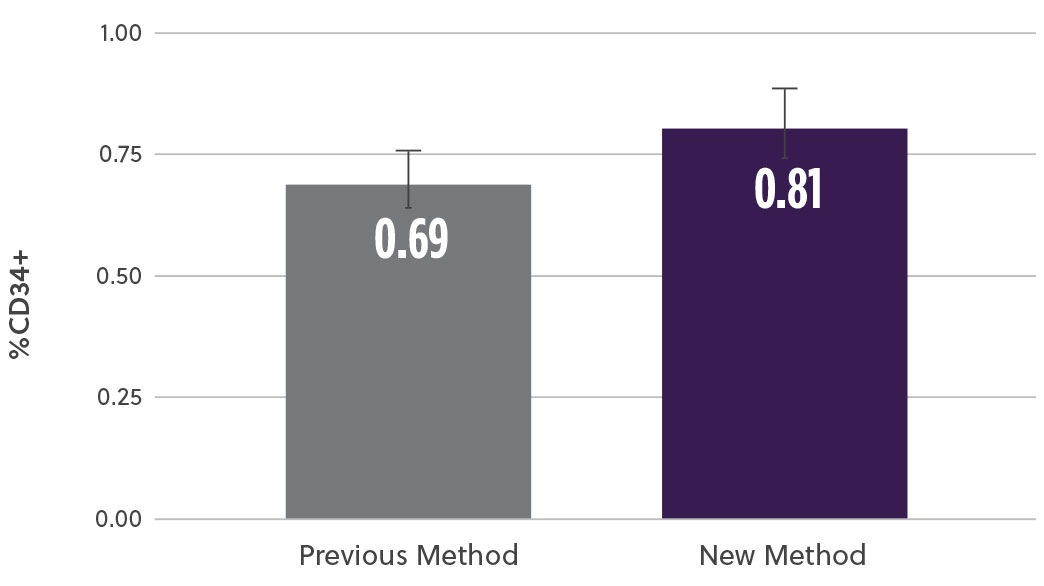
Fig 2c. Mononuclear Cells (MNCs) and Neutrophils
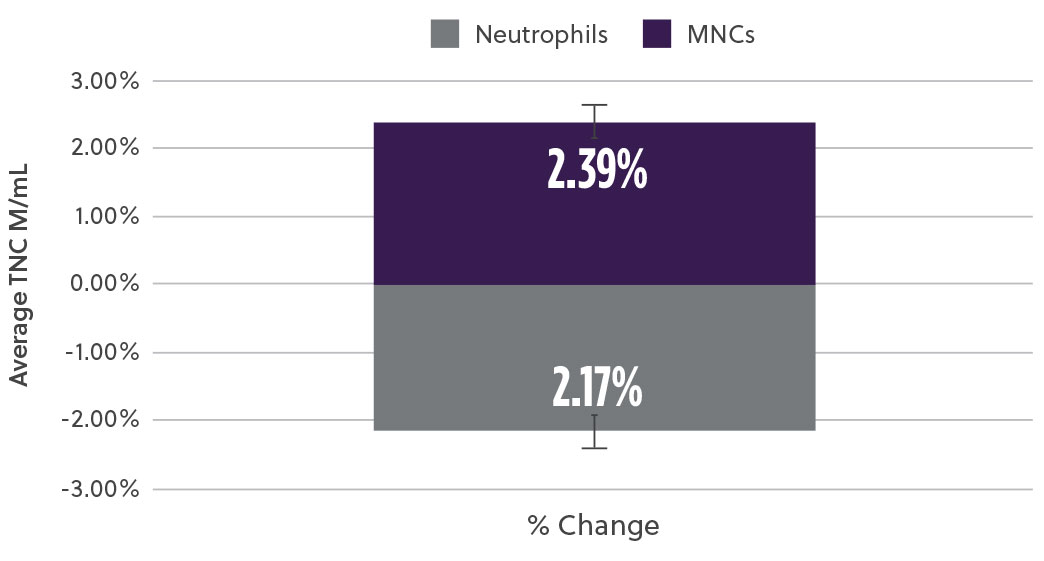
Fig 2. Bone marrow aspirate samples using the two methods were analyzed and compared for different subpopulations a) Average viable total nucleated cells (TNCs) was assessed using hematology analyzer b) viable %CD34 cells using ISHAGE method c) Viable mononuclear cells (MNCs) and neutrophils % change using a hematology analyzer
AllCells Bone Marrow Product Portfolio
Seamlessly Transition from Research to Clinical Trials & Commercialization with High-Quality, Consistent Cells
Bone marrow aspirate is collected into syringes containing heparin by our qualified clinicians from IRB-consented healthy human donors at our FDA-registered collection center. Fresh material is immediately processed at our facilities. Downstream cell isolation and/or cryopreservation is available. AllCells products undergo rigorous quality review to ensure adherence to minimum specifications prior to release. GMP product undergoes additional extensive quality review with extended documentation in accordance with FDA requirements.
Bone Marrow Aspirate Product Formats
| Research Use Only (RUO) Fresh | Research Use Only (RUO) Cryopreserved | GMP Fresh | |
|---|---|---|---|
| Whole | 3mL, 10mL, 25mL, 50mL, 100mL | — | 100mL |
| CD34+ PS | Custom, Bulk | 0.5M, 1M, 2M | — |

How Can We Help You?
Looking for research tips? Need help sourcing materials? How about a few more articles on therapies or processes? At AllCells, we believe that partnerships create the best pathways to success. Our Customer Success Team has years of industry experience and a wealth of resources to help you get the most out of your cellular products. We’re only a few clicks away.

